pH and Your Skin
Perhaps you’re familiar with pH and its function in daily life. If so, great! If not, continue reading for how important this two letter term is to our everyday life.
A pH is the measured amount of hydrogen ions present in a solution. The amount of hydrogen ions determine whether a solution is acidic or basic (alkaline). The pH scale ranges from 0-14 (acidic/basic), with 7 being neutral. Anything with water has a measurable pH, including our skin.
The skin’s pH is determined by the acid mantle. As discussed in Skin Fundamentals 101, the acid mantle is what is responsible for maintaining healthy, balanced skin by providing protection. The acid mantle is composed of several naturally occurring goodies, such as lactic acid. and ceramides. The acid mantle provides protection to the underlying layers of the skin. Outside elements, bacteria and debris do not stand a chance passing through. That is, if it is healthy and uncompromised.
The interesting thing about pH is that it is variable and constantly changing or adjusting. The acid mantle is acidic (hence its name) with a pH of 4.5 - 5.5. There are several factors that can raise or lower the acid mantle’s pH. This can include internal or external factors. More commonly, it is what we use on our skin that has the most effect.
For example, if I were to use a bar of Dial soap to wash my face and body, I would be left with dry, tight skin all over. The pH of the standard lye based bar soap is very alkaline (approx 9-10). Lye (sodium hydroxide) is the main component in bar soaps that turn the oils into a soap. Using a product with an alkaline pH will raise the pH of acidic skin resulting in the dry, tight feeling. Overtime, these symptoms tend to continue and get progressively worse.
Water alone can also disrupt the balance of the acid mantle due to its neutral nature.
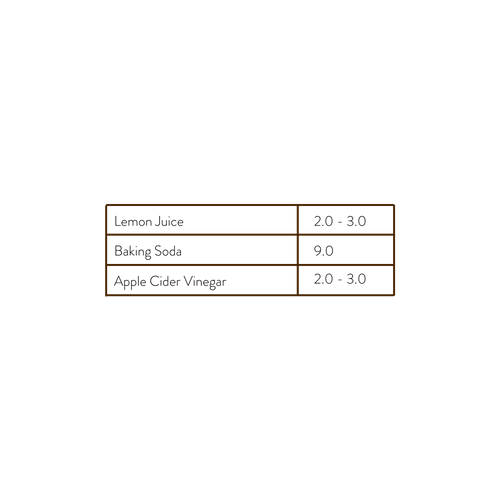
The DIY products trend has become increasingly popular in its presence online. From videos to tutorials, there are several resources to find easy to make solutions for skincare issues. But are these actual solutions? Before you go knee deep diving into your kitchen cabinet, I want to provide some warning. As stated above, every product or ingredient that is water based will have a pH. This does not include oils and other emollients, such as olive oil, grapeseed oil, etc. Let’s take a look at the pH’s of some of the most popular skincare concoctions I’ve come across:
The ingredients listed in the chart above are ones that I come across frequently and cringe at every time. Lemon juice and any of the other citruses (orange, grapefruit, lime, etc.) are very acidic ingredients. This is also true for apple cider vinegar or white vinegar. Using these alone can lead to increased sensitivity and over exfoliation. The citrus family are notorious for increasing sun sensitivities as well. Baking soda is very alkaline. The powder itself, even diluted with water, is too basic to use on our skin. I highly advise against using these types of ingredients to avoid further damage or burns.
If brightening or lightening of dark spots is what you’re trying to achieve, look for a product with lightening agents, such as licorice root and vitamin C.
If you are seeking exfoliation, the use of alpha hydroxy acids or enzymes are the best bet.
Curious about the pH of your favorite products? Try testing them yourself!
You can purchase these handy pH test strips online for a reasonable price. They honestly don’t work as well on very thick, creamy products, however, it’s worth the shot for other types.
If hands on isn’t your thing, looking at a product’s ingredient list, name, and description can normally give you an idea of the product’s pH range. Descriptions like exfoliating and brightening, or ingredients with alpha hydroxy acids (lactic, glycolic, mandelic, malic, citric, tartaric acid(s)) will more than likely be on the acidic side.
Were you aware of pH and its importance to your skin? If not, how will you use this information going forward?